Microscopic bits of metal – nanoparticles, a thousand times smaller than the thickness of a human hair – have been touted as a biotechnology and manufacturing miracle. Among many other applications, they can be used to keep the smell out of sweaty gym clothes, to treat wastewater, and they are being considered as a way to deliver cell-specific cancer drugs. However, as a recent experiment shows, the UV from sunlight that make nanoparticles so valuable in killing harmful and unpleasant bacteria also kill ocean phytoplankton that regulate the climate and are the base of the oceans’ food chain.
The study – whose lead author is Robert Miller of the Bren School of Environmental Science and Management at University of California Santa Barbara – was published on January 20, 2012 in the online journal PLoS One. It is called “Titanium Dioxide (TiO2) Nanoparticles Are Phototoxic to Marine Phytoplankton.” Miller and his colleagues conducted an experiment showing that even normal levels of ultraviolet light (UV) from sunshine are enough to cause titanium dioxide nanoparticles suspended in seawater to kill phytoplankton.
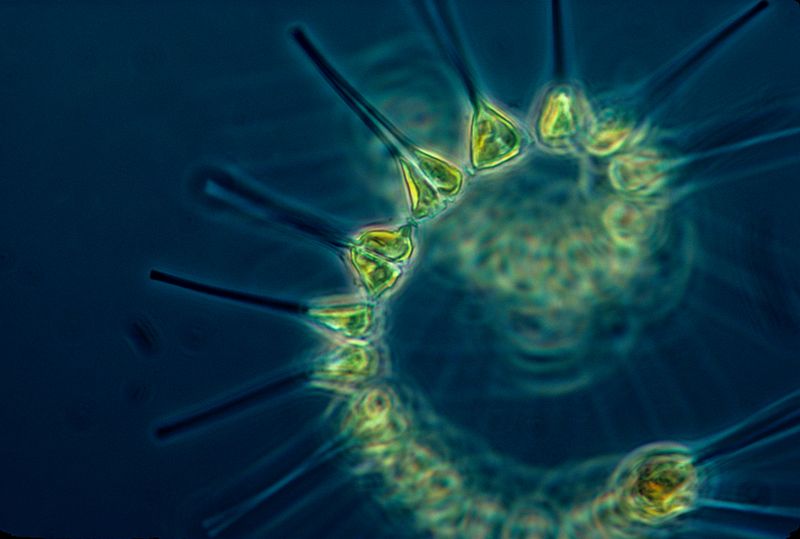
Phytoplankton are very small marine organisms (most are too small to be seen with the unaided eye) that regulate the global climate by taking up vast quantities of carbon dioxide, or CO2, from the atmosphere via photosynthesis. They also serve as the first link in the oceans’ food chain. Phytoplankton that are not eaten by other marine creatures end up sinking into the deep ocean, which is one of the only ways carbon is taken out of the atmosphere and naturally stored for thousands of years.
Titanium dioxide is by far the most commercially used nanoparticle. About 70% of all pigments use titanium dioxide, and it is a common ingredient in products such as sunscreen and food coloring. Titanium dioxide is therefore likely to enter estuaries and oceans, for example from industrial discharge, and new studies such as Miller’s have focused on measuring the impact of metal nanoparticles on marine life like phytoplankton.
Nanoparticles are highly reactive with oxygen after exposure to ultraviolet (UV) light. This reaction gives nanoparticles significant antimicrobial properties, which is why they are effective against your smelly gym clothes.
Meanwhile, nanoparticles suspended in water can easily attach to phytoplankton cell membranes. Damage to plankton occurs when titanium dioxide is electronically excited due to energy from UV light. Electrons from the nanoparticle react with surrounding water to form reactive oxygen species (ROS), which are very potent oxidizers that damage cell membranes and degrade proteins and organic compounds within a cell.
It is well established that titanium dioxide – as well as zinc oxide (ZnO) – exposed to artificially high levels of UV is electronically excitable and toxic to cells. However, the experiment by Robert Miller and his colleagues showed that normal levels of UV from sunshine can excite titanium dioxide nanoparticles suspended in seawater, kill phytoplankton, and lead to population crashes of some phytoplankton species in a laboratory setting.
While nanoparticles are thought not to be entering marine ecosystems yet in large quantities, this experiment is proof of concept that phytoplankton populations are highly vulnerable to damage from nanoparticles.
In an email, Dr. Miller told EarthSky that:
This could mean less support for ocean food webs, which includes fisheries and marine mammals. It could also impact the global carbon cycle – less phytoplankton production could mean less CO2 taken out of the atmosphere, and less carbon sequestered on the ocean floor.
They (nanoparticles) could aggregate with each other and with natural particles and settle to the seafloor, where they would affect bottom-dwelling organisms. We’re also working on that question.
Dr. Lucas Thompson, an Assistant Professor of Chemistry at Gettysburg College and an expert in metal nanoparticle applications, but not an author on Miller’s study, stated that nanoparticles still hold tremendous promise with some caveats. He said:
These nanoparticle interactions can be harnessed to fight cancer or to help deliver drugs in a controlled manner.
However, it must be noted that these same interactions can prove to be toxic if not carefully managed. This study highlights some of the potentially devastating effects that careless disposal of nanomaterials could have on the marine environment.
Bottom line: An experiment led by Robert Miller of the University of California Santa Barbara indicates that even normal levels of ultraviolet light (UV) from sunshine are enough to cause titanium dioxide nanoparticles suspended in seawater to kill phytoplankton, in a laboratory setting. The study – called “Titanium dioxide (TiO2) Nanoparticles Are Phototoxic to Marine Phytoplankton” – was published on January 20, 2012 in the online journal PLoS One.
Submit your own Earth or night sky photos at EarthSky Community Photos.











